Introduction
Patients with breast cancer (BRCa), the leading cause of death from cancer in women in Mexico (Knaul, López-Carrillo, Lazcano-Ponce, Gómez-Dantés, Romieu, & Torres, 2009; GLOBOCAN, 2012), may concomitantly face the risk of a metabolic syndrome (RSxM), which includes the presence of certain highly prevalent diseases, such as obesity, arterial hypertension, and/or type II diabetes mellitus (Mohar, Reynoso, Villareal-Garza, Bargalló-Rocha, Arce-Salinas, & Lara-Medina, 2015). This comorbidity not only increases harm but also physical and psychological demands, which, if not properly treated, worsen the evolution of both illnesses (Lifshitz, 2016; National Institute on Drug Abuse [NIDA], 2017). It has been found that physical and psychological demands cause women with oncological or metabolic diseases to perceive themselves as being stressed during the development of the disease (Lebel, Rosberger, Edgar, & Devins, 2007). In these patients, stress has been evaluated through the recording of peripheral physiological responses and the use of psychometric instruments (subjective recording).
Physiological evaluations show that, when patients with BRCa face stressful situations, they respond with high cardiovascular, muscular, and electrodermal activity, in comparison with their baseline (Karvinen, Murray, Arastu, & Allison, 2013; Bower, Ganz, & Aziz, 2005; Pitman, Lanes, Williston, Guillaume, Metzger, Gehr et al., 2001; Watson, Pettingale, & Greer, 1984). Conversely, patients with RSxM (without diabetes) present, from heir baseline, a high cardiovascular, muscular, respiratory, and peripheral temperature, which increases in response to the stressful stimulus (Garafova, Penesova, Cizmarova, Marko, Vlcek, & Jesova, 2014; Kaushik, Mahajan, Rajesh, & Kaushik, 2004; Pasquali, Anconetani, Chattat, Biscotti, Spinucci, Casimirri et al., 1996; Fredikson, Dimberg, Frisk-Holmberg, & Ström, 1982). In the subjective evaluation, patients with BRCa reported moderate stress levels (Becerril, Camacho, & Canabal, 2013), whereas women in the RSxM group reported the highest stress levels, compared with men (Pasquali et al., 1996; Ramírez, 2005; Delgado, 2010).
The relevance of these findings is based on two facts. The first is that the greater the stress, the lower the quality of life perceived by patients with BRCa (Hernández, 2012) during active treatment, coupled with the negative effect of the secondary physical reactions of cancer treatments (such as pain, fatigue, and constipation). The second is that chronic stress causes anxiety, depression, and fatigue (Hernández, 2012; Schunk, Reitmeir, Schipf, Völzke, Meisinger, Ladwig et al., 2015; Kirk, Price, Penney, Rehman, Lyons, Piccinini-Vallis et al., 2014).
If we consider that the literature has found, separately, that both groups of patients experience physical and psychological reactions to stressful situations that may put their adaptation to the disease and their everyday lives at risk, and that patients with comorbidity may differ in this regard from those with only one condition, it is therefore necessary to determine:
-
Whether patients with comorbidity are different from those who do not have it in terms of their stress response (physiological and psychological), to identify whether comorbidity alters the stress response as hypothesized, and also, whether this group requires specific interventions for this reason.
-
What the physiological and psychological stress responses are like simultaneously in order to confirm the conceptual components of the stress response, as well as to determine whether it is only perceived or whether there is a physiological reaction, which will determine the clinical training that will be designed.
-
Whether the groups have already altered stress responses before receiving oncological interventions. In this case, this would justify the need for psychological therapy from the time of the recent diagnosis in order to reduce the effect of the first interventions in these terms.
Accordingly, the objectives of this study are to evaluate the physiological and psychological differences between the BRCa-RSxM groups and those with BRCa alone, and the influence of psychological variables and comorbidity in terms of the stress response.
We hypothesize that patients with BRCa-RSxM will have significantly higher levels of physiological and psychological stress.
Method
Design
Comparative non-experimental causal-descriptive study.
Participants
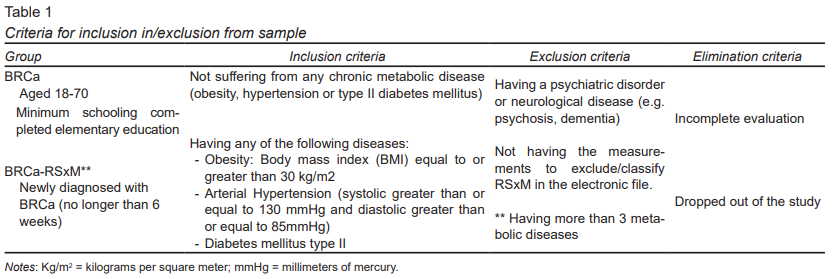
We recruited recently-diagnosed BRCa patients ages 18 to 70. They were classified into two groups: those with BRCa alone and those with BRCa-RSxM. The inclusion, exclusion, and elimination criteria are detailed in Table 1.
The study population was recruited through convenience sampling in the Department of Breast Tumors of the Instituto Nacional de Cancerología (INCan) of Mexico City, from August 2016 to June 2017.
Instruments
The following selected psychometric instruments have been validated in the Mexican population:
-
Perceived stress. Perceived stress was evaluated using the Perceived Stress Scale (Cohen, Kamarck, Mermelstein, 1983). This type of stress is conceptualized as the degree to which life situations were perceived as stressful in the past month. It consists of 14 items, with four response options (0-4) in a Likert-type format. Scores range from 0 to 56: the higher the score, the greater the degree of stress. The version for Mexican population reached a Cronbach’s alpha of .83 (González Ramírez & Landero Hernández, 2007).
-
Quality of life. In keeping with the European Organization for Research and Treatment of Cancer-Quality of Life (EORTC-QLQ), the QLQ-C30 and its supplementary module, QLQ-BR23, were applied. Both instruments evaluate aspects of the disease and symptoms related to oncological treatment, and physical, psychological, and social functioning in order to obtain relevant, detailed information.
-
General module. QLQ-C30 (Aaronson, Ahmedzai, Bergman, Bullinger, Cull, Duez et al., 1993; Oñate-Ocaña, Alcántara-Pilar, Vilar-Compte, García-Hubard, Rojas-Castillo, Alvarado-Aguilar et al., 2009). Instrument with 30 items, and four Likert-type response options, divided into multi-item scales: five functional scales; three symptom scales and one overall health scale; and six scales with a single item. In its version for the Mexican oncological population, it reached a Cronbach’s alpha of > .70 in all the scales.
-
Breast cancer module QLQ-BR23 (Sprangers, Groenvold, Arraras, Franklin, te Velde, Muller et al., 1996; Cerezo, Oñate-Ocaña, Arrieta-Joffe, Gonzalez-Lara, Garcia-Pasquel, Bargalló-Rocha et al., 2012). Specific for patients with breast cancer at any clinical and treatment stage. This module consists of 23 questions, four Likert-type response options, indicating the frequency of functionality or symptoms. It is divided into five multi-item scales, and three single-item scales. In the version for the Mexican cancer population, it obtained a Cronbach’s alpha of .52 -.70.
For both instruments, the minimum score is 0 and the maximum score 100. A high score on the functionality and quality of life scales represents high levels of quality of life, whereas a high score on the symptoms scale means a high level of symptoms or problems (Fayers, Aaronson, Bjordal, Groenvold, Curran, Bottomley et al., 2001).
-
Coping strategies. Coping strategies were explored using the Cognitive Emotional Regulation Questionnaire (Garnefski, Kraaij, & Spinhoven, 2002), which conceptualizes them as thinking strategies used by the persons to regulate themselves emotionally in the face of unpleasant or stressful experiences (Compas, Orosan and Grant, 1993). It is a 20-item instrument with five Likert-type response options, divided into two categories of strategies: 1. adaptive and 2. maladaptive. The higher the score, the more a strategy is used. In the version for the Mexican oncological population, it obtained a Cronbach’s alpha of .78 and an explained variance of 57.2% (Gálvez-Hernández, Rivera-Fong, Linares-Buitrón, Zapata-Barrera, Mohar-Betancourt, Calleja-Bello et al., 2018).
The physiological variables registered (with computerized equipment from J and J Engineering I-330 - C2 +) were as follows:
-
Muscle activity of front muscles (Cram, Kasman, & Holtz, 2011) recorded by means of surface electromyography (EMGs). It is conceived as the sum of potentials of an indeterminate set of motor units, located in the front muscle, expressed in micro volts (mvolts). Higher levels of mvolts are interpreted as higher levels of activation.
-
Conductance of the skin in the hand (Dawson, Schell, & Filion, 2007) registered through the electrodermography technique (EDG) as a change produced in the conductance caused by an externally applied current. It is expressed in microSiemens (mS). The higher the mS levels, the higher the activation levels.
Procedure
The study was approved by the Ethics and Research Committees of INCan (016/033/IBI, CEI/1045/16). First of all, psychologists trained in psychophysiological evaluation used clinical information in electronic files to find patients who met the inclusion criteria. The day they went to the Institute to receive their BRCa diagnosis for the first time, while they were in the waiting room, they were invited to participate and had the study explained to them. If they accepted, they signed the informed consent form.
The evaluation was carried out in a single session. First of all, the psychological part was performed, which included a clinical interview, prepared ex profeso, which collected sociodemographic data, clinical data (oncological and metabolic), and information on the respondents’ lifestyle (sleep habits, physical activity, and diet), as well as the application of psychometric instruments.
Afterwards the physiological evaluation was carried out. This consisted of placing EMG electrodes on the area of the forehead, and EDG electrodes on the distal phalanx of the ring finger of the left hand (Khazan, 2013; Arena & Schwartz, 2003). During the entire evaluation time, patients remained connected to the sensors and sat in front of a computer, where they were given instructions for each of the following conditions (which lasted two minutes and were continuous): 1. adaptation period (familiarize the patient with the evaluation); 2. baseline 1 (neutral conversation); 3. cognitive stress (counting down in sevens from 1000); 4. recovery 1 (they remained seated); 5. emotional stress (verbal recollection of a stressful event); 6. recovery 2; 7. baseline 2 (they sat silently), 8. relaxation (in the way they usually do), and 9. recovery 3. After the registration had been completed, the patient’s perception of these conditions was explored.
Statistical analysis
The physiological data statistically analyzed were obtained from the raw signal recorded and analyzed by the equipment used. It uses a 100 to 400 Hz filter to eliminate artifacts, and a digital filter that allowed the transmission of 60 Hz to the computer in the form of a raw wave. The program rectified and integrated the signal to provide an area under the curve and was also transformed using a Fast Fourier transform (Engineering, J & J, 2004).
First of all, this was used to undertake a descriptive analysis (with central tendency and dispersion measures for continuous variables, and frequencies and percentages for categorical variables). Secondly, normality (Shapiro-Wilk) and homoscedasticity (Snedecor’s F) tests were carried out. Thirdly, in order to respond to objective 1, a bivariate analysis was performed where the BRCa and BRCa-RSxM groups were compared, using the statistical test by type of variable: Student’s t or Mann Whitney U (numerical variables) and Chi-square or Fisher’s exact (categorical variables). Fourth, for objective 2, the average of each physiological variable was calculated for each evaluation phase. Multiple linear regression models and nonlinear models (quantile) were proposed, according to the behavior of the variable, to establish the relationship between physiological variables (dependent variable) and the other variables (called predictors: presence of RSxM, overall quality of life, perceived stress, quality of social life, perspective, positive approach, emotional focus, and insomnia) during stress tasks. In other words, in order to evaluate muscle activity and conductance in stressor conditions, quantile regressions were presented, expressing the quantiles of conditional distribution as linear functions of independent variables. Their robustness was an advantage compared to the extreme values obtained in the determinations (Vicéns & Sánchez, 2012). Four models were presented: 1. muscle activity with emotional stressor (linear regression); 2. Muscle activity with cognitive stressor (nonparametric regression); 3. Skin conductance with emotional stressor (non-parametric regression) and, 4. Skin conductance with cognitive stressor (nonparametric regression). All the models were adjusted for the variables presented in Table 2, and by age and clinical stage. Coefficients, 95% confidence interval (95% CI), and p values are presented, considering values of ≤ .05 to be statistically significant. All analyses were performed using STATA 14.2.
Results
Sociodemographic and clinical characteristics
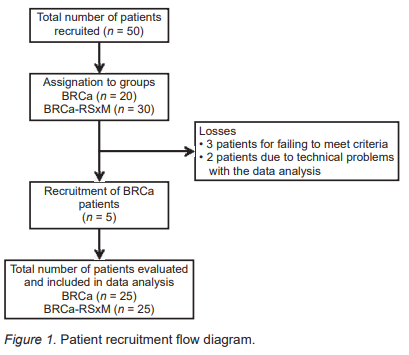
Fifty-five women with a confirmed BRCa diagnosis were recruited, five of whom were excluded (three patients after the evaluation showed that their clinical data failed to meet the inclusion criteria, and two due to technical problems in the data analysis). The final sample comprised 50 patients, divided into two groups: 25 with BRCa and 25 with BRCa and RSxM (Figure 1).
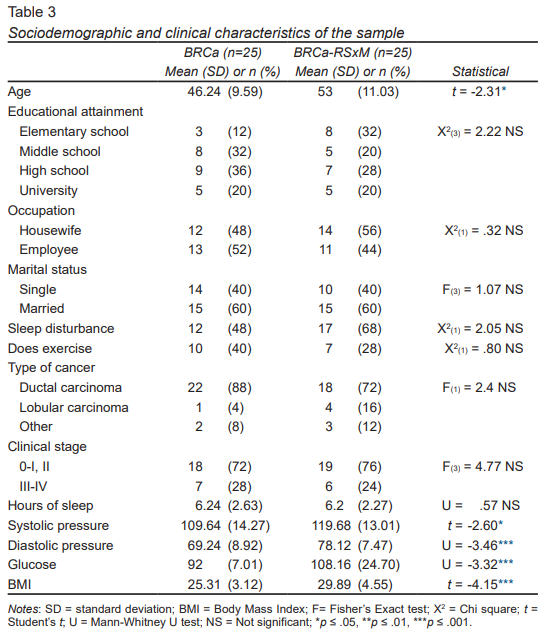
The BRCa group was significantly younger (45 years) than the comorbidity group (52 years) (t = -2.31, p ≤ .05). Most of the patients with BRCa had completed high school (36%) and were employed (52%), while the BRCa-RSxM group had finished elementary school (32%) and were housewives (56%). Both groups of patients were married and the majority were Mexico City residents ( Table 3).
The groups were similar as regards lifestyle, sleeping habits, and physical activity. Most patients in both groups had ductal carcinomas and 50% of the women in each group had stage II cancer. The physiological values of comorbidity were significantly higher (p < .05) in BRCa-RSxM; on average, they had had 3.2 years of evolution of the disease.
Physiological and psychological differences between groups in stressful situations
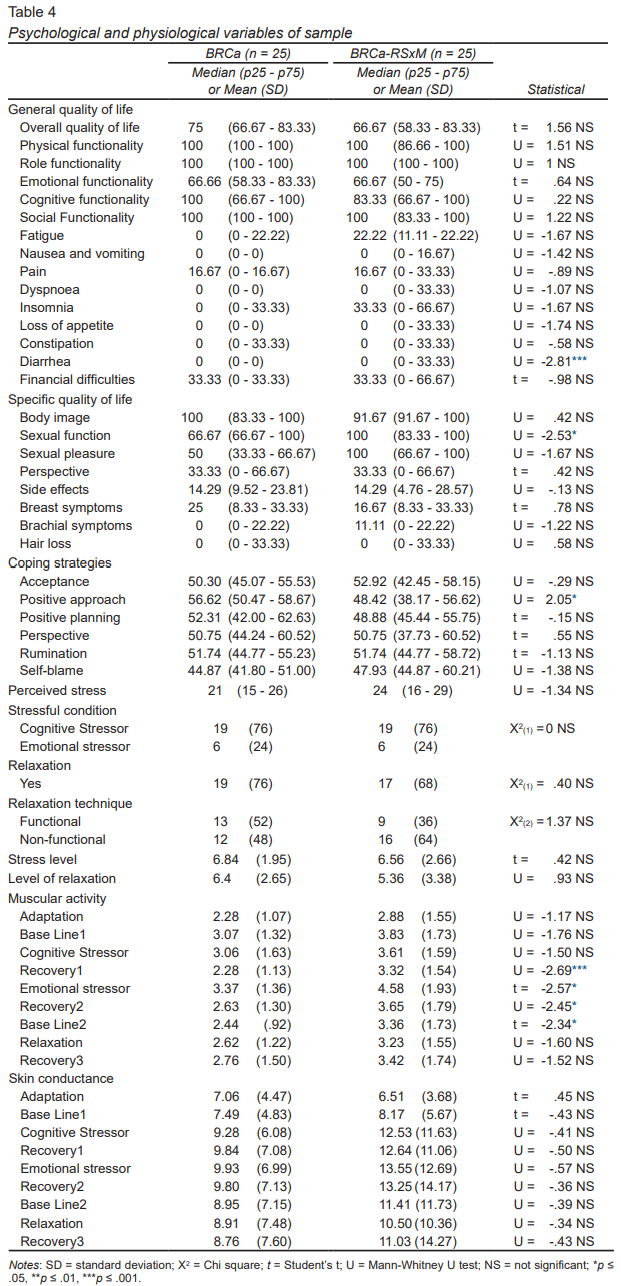
Muscle activity in the two groups was similar during baseline 1 and the cognitive stressor. The BRCa-RSxM group showed significantly higher levels of muscle activation during recovery 1, emotional stressor, recovery 2, and baseline 2 (U = -2.69, p ≤ .01, t = -2.57, p ≤ .05, U = -2.45, p ≤ .05, t = -2.34, p ≤ .05, respectively). The greatest difference was observed in the emotional stressor (BRCa-RSxM = 4.58 [1.93], BRCa = 3.37 [1.36]).
Both reported moderate levels of perceived stress but the BRCa group reported higher levels of overall quality of life and physical and emotional functioning, without significant differences. Women with BRCa-RSxM reported significantly more symptoms in diarrhea (U = -2.81, p ≤ .01), and better sexual function (U = -2.53, p ≤ .05). The BRCa group used the positive approach more significantly as coping strategies (U = 2.05, p ≤ .05) ( Table 4).
Influence of psychological variables and comorbidity to stressful situations
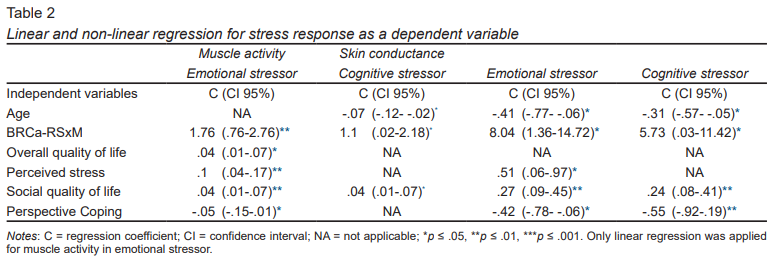
According to the multivariate model for physiological assessment during stressful situations ‒ cognitive and emotional ‒ ( Table 2 ), the only values with a normal distribution were those for muscle activity during the emotional stressor, as a result of which a multiple linear regression was proposed.
Muscle activity during the emotional stressor, in the group with BRCa-RSxM, was 1.76 mvolts higher, in comparison with the group without comorbidity (C = 1.76, CI 95% [.76, -2.76], p ≤ .01). Muscle activity increased .1 mvolts for each perceived stress point (C = .1, CI 95% [.04, -.17], p ≤ .01), and .04 mvolts for each 1 unit increase on the Likert scale of overall quality of life (C = .04, CI 95% [.01, -.07], p ≤ .05) and quality of social life (C = .04, CI 95% [.01, .07] p ≤ .01) ( Table 2 ). Conversely, for each point of increase in frequency of use of the “perspective” coping style, muscle activity was reduced by .05 mvolts (C = -.05, CI 95% [-.15, -.01], p ≤ .05).
During the cognitive stressor, women with BRCa-RSxM presented .1 mvolt of greater muscle activity (C = 1.1, CI 95% [.02, -2.18], p ≤ .05); as well as .04 mvolts for each .01 increase in the intensity of presence of quality of social life (C = .04, CI 95% [.01, -.07], p ≤ .05). For each one-year increase in age, activity decreased by .06 mvolts (C = -.07, CI 95% [-.12, -.02], p ≤ .05).
Cognitive stressor conductance was five times higher in women with BRCa-RSxM (C = 5.73, CI 95% [.03, -11.42], p ≤ .05). In addition, for each 1-point increase in the presence of quality of social life, conductance increased .24 mS (C = .24, CI 95% [.08, -.41], p ≤ .01), and decreased .03 units for every one-year increase in age (C = -.31, CI 95% [-.57, -.05], p ≤ .05), and .55 for each point of increase in the frequency of use of perspective coping (C = -.55, CI 95% [-.92, -.19], p ≤ .01). Conductance during the emotional stressor showed a greater difference between the groups (C = 8.04, CI 95% [1.36, 14.72], p ≤ .05), with similar results to those of the cognitive stressor, as regards age and “perspective” coping style. Finally, for every increase in perceived stress and quality of social life, conductance increased by .51 and .27 mS (C = .51, CI 95% [.06, -.97], p ≤ .05; C = .27, CI 95% [.09, -.45], p ≤ .01, respectively) for this emotional condition.
Discussion and conclusion
The present study evaluated differences in the physiological and psychological response (in quality of life, perceived stress, and use of coping strategies) in stressful situations, among groups of patients, as well as the influence of these psychological variables and the disease (and/or comorbidity) in the physiological response to stressful situations.
Physiological and psychological differences between groups in stressful situations
Patients with comorbidity displayed significantly higher physiological activation, used certain adaptive coping strategies less frequently, and had similar levels of overall quality of life, yet better sexual function.
The high levels of muscle reactivity in the BRCa-RSxM group are consistent with the population with RSxM without cancer (Kaushik, Mahajan, Rajesh and Kaushik, 2004).
This may be due to comorbidity due to the increase or balance in the activity of the hypothalamic-pituitary-adrenal axis (Pasquali et al., 1996) or greater sympathetic innervation (Esler, Rumantir, Kaye, Jennings, Hastings, Socratous et al., 2001). It may also be due to the fact that 72% of the BRCa-RSxM group did not do exercise, a practice which creates less intense responses to stressors and faster recoveries (Stulrajter, Scholzova, Sleboda, Dlhos, & Miklanek, 1997).
The fact that patients with BRCa used the positive approach as a coping style more frequently coincides with several studies (Li, Zhu, Yang, He, Yi, Wang et al., 2015; Becerril et al., 2013; De Haro-Rodríguez, Gallardo-Vidal, Martínez-Martínez, Camacho-Calderón, Velázquez-Tlapanco, & Paredes-Hernández, 2014). This may have been because the BRCa-RSxM group was significantly older, since it is related to less use of adaptive strategies (De-Haro-Rodríguez et al., 2014).
The similarities between the groups as regards quality of life underscore the fact that suffering from metabolic disease does not affect this perception (Sat-Muñoz, Contreras-Hernández, Balderas-Peña, Hernández-Chávez, Solano-Murillo, Mariscal-Ramírez et al., 2011). This may be due to the fact that they still did not have health complications (years of evolution: 3.2 ± 6.3 years), they were controlled, and/or had not been recorded by the instrument built to evaluate the effects of cancer treatment.
Influence of psychological variables and comorbidity on stressful situations
Comorbidity and the quality of social life influenced the increase in activation, whereas age and coping decreased it.
The influence of comorbidity could act as a form of physiological vulnerability (Dubovsky, 1985), produced by the metabolic alterations and sedentary lifestyle that characterized patients. The quality of social life was understood as a reflection of the evaluation of the stressful stimulus, which results in the use of social support, which requires behavioral and emotional activation (reflected in muscle activation and conductance).
The decrease in physiological activation was understood as a result of patients’ “decreasing” the severity of the event (by comparing it with worse ones) during the emotionally stressful situation (Garnefski et al., 2002). This notion coincides with the fact that greater use of maladaptive strategies and emotional suppression increases physiological activation (Giese-Davis, Conrad, Nouriani, & Spiegel, 2008). Conversely, the influence of increasing years of age during the cognitive stressor may be a habituation response (Andreassi, 2007). In other words, patients optimized their energy in response to the stressors ‒ decreasing activation ‒, and the arithmetic task, which were familiar to them (as a result of their age and schooling).
Physiological vulnerability and the behavioral coping strategy increased activation levels. At the same time, cognitive coping strategies and previous learning were related to the decrease in physiological activity, which, in turn, depended on the type of stressor.
The discussion of these results may be limited by the lack of previous studies with which to contrast them. Uncontrolled variables may have influenced the results obtained: the evaluators’ knowledge of the study hypothesis, the time since the diagnosis and the use of medicines by comorbid patients.When the sample increase was explored in one group, no significant differences were observed, or in the data trend.
In this study, we found that the BRCa-RSxM group was found to be physiologically more reactive to stress, and psychologically similar as regards perceived stress, quality of life, and coping strategies to the BRCa group. Physiological vulnerability, coping strategies (behavioral and cognitive), and prior learning influenced the resulting reaction produced during the stressful situation. This supports the fact that the full understanding of the relationship of stress with health-disease processes requires incorporating the conceptualization of a complex interaction of biological, psychological, and social variables, confirming the theoretical proposals of Dubovsky (1985) and Sandín (1995).
When exploring the physiological and psychological stress responses ‒ recorded simultaneously ‒ we confirmed that they co-occur both positively (muscle activity and conductance with perception of stress) and negatively (coping styles with muscle activity and conductance) in response to exposure to a stressful situation. In both groups, the physiological pattern coincides with perception. The study also shows that this response is not altered in the recent diagnosis of oncological disease or in metabolic comorbidity.
However, the differences found show that suffering from a metabolic disease, as a prelude to an oncological disease, could cause physiological vulnerability which, in turn, predisposes a person to respond inadequately to stressful conditions. This is compounded by the effects of an inadequate cognitive evaluation of resources (social and personal), and the type of stressor.
Clinically, the results support the usefulness of interventions in newly diagnosed BRCa-RSxM patients, designed to modify the physiological reactions to events regarded as stressful. At a psychological level, the results show that the type of stressor, social-behavioral, and cognitive resources can be important therapeutic components.
Knowing how both groups of patients respond to stressful situations in the recent diagnosis is unprecedented; however, this study confirms that they perceive themselves as being stressed from the start of the cancer trajectory. It also makes it possible to determine the advisability of implementing interventions of a psychophysiological nature at this stage, such as biofeedback. This could help patients provide better adapted responses to future stressful situations, such as active treatment.